Advantages
(1) Predicting Efficacy and Stratifying Patients for Combination Immunotherapy: Pre-treatment plasma sTWEAK levels can predict the response to Durvalumab/Tremelimumab therapy, enabling informed decisions on its use versus Atezolizumab/Bevacizumab therapy.
(2) Potential for Expansion into a Panel Test: This biomarker can serve as a foundation for a comprehensive drug selection panel test. By combining it with other biomarkers, the panel could guide the use of not only combination immunotherapies but also multi-kinase inhibitors.
Background and Technology
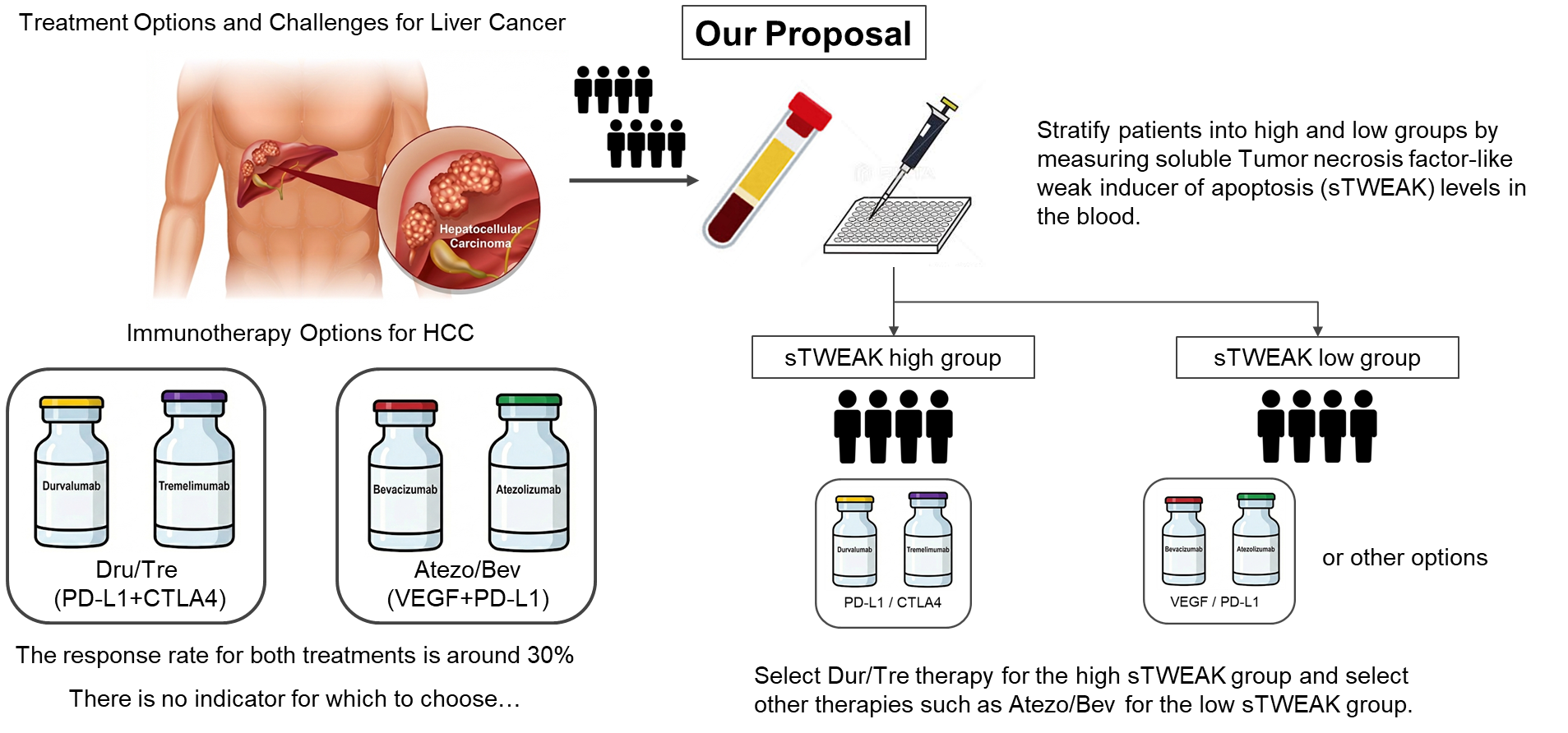
Unmet Clinical Need: While Atezo/Bev and Dur/Tre are standard first-line therapies for advanced HCC, their response rates are limited (20–30%), creating an urgent need for biomarkers to guide the selection of the most effective regimen for each patient.
Identification of sTWEAK: Using high-throughput proteomics on data from a multi-center study, researchers identified soluble TWEAK (sTWEAK) in the blood as a significant biomarker correlated with the therapeutic effect of Dur/Tre therapy.
Predictive Value for Dur/Tre: Patients with high pre-treatment plasma sTWEAK levels demonstrated significantly longer progression-free survival (PFS) and overall survival (OS) when treated with Dur/Tre.
Treatment Stratification: Conversely, for patients with low sTWEAK levels, Atezo/Bev therapy may lead to better overall survival than Dur/Tre, making sTWEAK a critical tool for differentiating between these two standard options.
Scientific Rationale: Mechanistically, high sTWEAK levels indicate an "immune hot" tumor microenvironment characterized by active T-cell infiltration, explaining why the dual immune checkpoint blockade of Dur/Tre is effective in this subgroup.
Practical Application: This biomarker can be measured using a simple, non-invasive ELISA method, facilitating personalized medicine that improves patient outcomes and offers economic benefits.
Key Data
Utility as a Treatment Selection Marker: High sTWEAK levels predict significantly extended Overall Survival (OS) with Dur/Tre therapy. Conversely, patients with low sTWEAK levels achieve superior OS with Atezo/Bev compared to Dur/Tre.
Expectations
Osaka University is actively seeking partnerships with diagnostic companies interested in this technology through licensing agreements or joint research collaborations.
We are open to collaboration on a wide range of themes, including validation in larger clinical cohorts, standardization of cut-off values, exploration of optimal combinations with other biomarkers, and investigation of sTWEAK as a potential new therapeutic target.
Upon signing a non-disclosure agreement (NDA), we can share unpublished data. Direct meetings with the researchers can also be arranged.
Principal Investigator
Takahiro Kodama, Assistant Professor (Osaka University Graduate School of Medicine)
Patents and Publications
Patent pending (not yet published)