Advantages
・Selectively cleavages mutant mtDNA in heteroplasmy, increasing the proportion of normal mtDNA
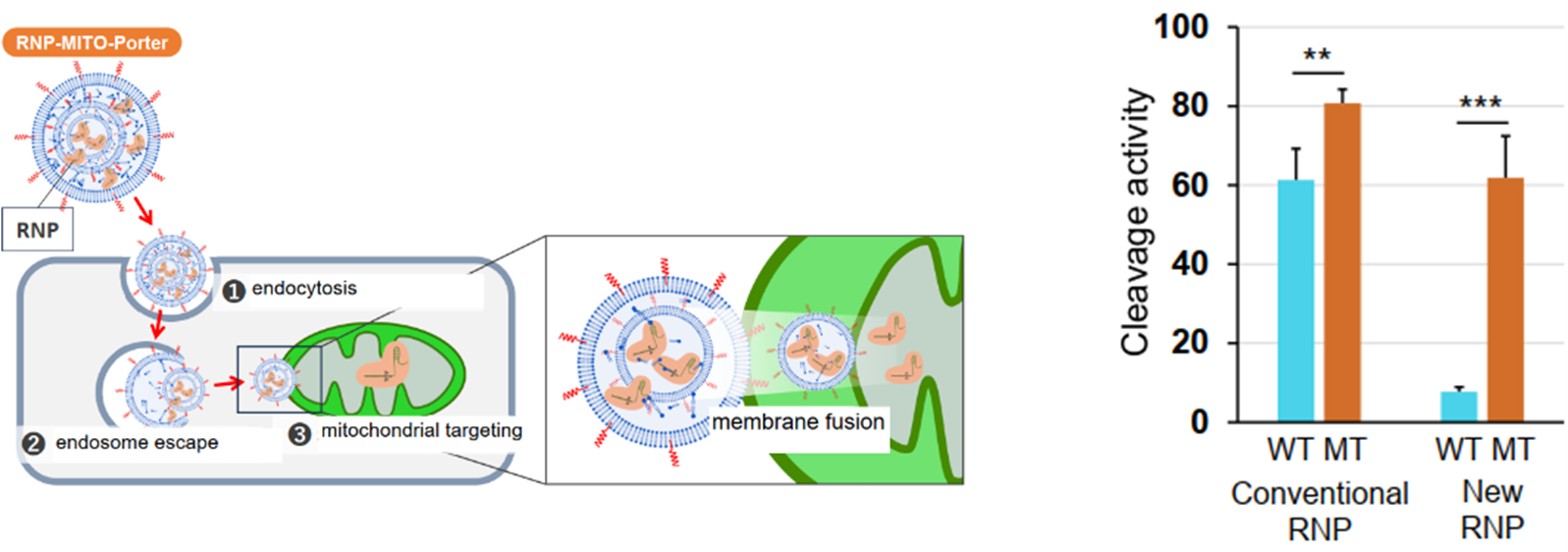
・RNP-MITO-Porter*1 can be used as a highly efficient RNP delivery technology to mitochondria.
Current Stage and Key Data
・The cleavage selectivity of the MELAS mutant mtDNA (m.3243A>G) by our new RNP was compared with that of a conventional RNP, and a five-fold improvement in selectivity and high cleavage activity were confirmed (in vitro).
Partnering Model
Seeking collaborative partners to develop therapeutic drugs for MELAS (m.3243A>G) using the RNP of this invention as a lead compound, or to work on genome editing drug discovery for other diseases using the design method of this invention.
・ Potential partners: mitochondrial disease treatment drug development companies, genome editing drug discovery companies, etc.
Background
The relationship between mitochondrial diseases and mtDNA mutations has been reported, and there is a need for radical treatment at the genetic level by genome editing targeting mutant mtDNA. However, the low efficiency of CRISPR/Cas9 system delivery into the mitochondria, which are surrounded by a strong bilayer membrane, has been an issue. We have previously reported a delivery technology called RNP-MITO-Porter*1 , which uses lipid nanoparticles (LNPs) to directly deliver RNPs into mitochondria.
The mitochondrial genome exists in several thousand copies per cell, and many mitochondrial diseases, such as MELAS, involve a heteroplasmy in which mutant and wild-type mtDNA coexist. As the proportion of mutant mtDNA increases, maintaining mitochondrial function becomes difficult, leading to the onset of disease. Therefore, eliminating mutant mtDNA using RNPs is effective, but conventional RNPs have had difficulty recognizing single-base differences in mutant mtDNA and cleaving it with high selectivity. The present invention provides an RNP that significantly enhances this selectivity.
Principal Investigator
Yuma Yamada, Prof. (Hokkaido University Graduate School of Pharmaceutical Sciences)
Patents and Publications
➢ References: *1 Norota et.al., Scientific Reports (2025) 15, 18717
➢ Patent pending (unpublished)