Advantages
- Tests using tumor cell-derived xenograft (CDX) mouse models and patient-derived xenograft (PDX) mouse models confirm efficacy in suppressing tumor volume growth.
- Specifically, in CDX mouse studies, combination with an immune checkpoint inhibitor resulted in significant tumor volume reduction (nearly complete disappearance).
Technology & Background
Cancer-associated fibroblasts (CAFs) play a pivotal role in the tumor microenvironment by secreting growth factors that drive cancer cell proliferation, remodeling the extracellular matrix, and facilitating invasion and metastasis. Furthermore, CAFs contribute to immune evasion by suppressing immune cell functions, thereby hindering the host's anti-tumor response. Consequently, CAFs have emerged as a high-potential therapeutic target, fueling the development of innovative CAF-targeted modalities. For example, modulating CAF phenotypes or integrating CAF-targeted nanoparticles and chimeric antigen receptor (CAR)-T cell therapies with conventional standards of care has demonstrated significant potential to enhance the efficacy of chemotherapy and radiotherapy.
Leveraging pancreatic cancer scRNA-seq data, researchers identified fibroblast activation protein (FAP) as a highly specific surface marker for CAFs. This finding paved the way for the development of a CAR-T cell therapy engineered to precisely target FAP. Through rigorous optimization, the team identified a potent mRNA sequence for a human FAP-specific CAR. By delivering this CAR mRNA via specialized lipid nanoparticles (LNPs) in combination with various oncology agents, they achieved significant tumor regression in preclinical mouse models.
Current Stage & Key Data
- Researchers designed CAR mRNA targeting FAP and introduced it into T cells via electroporation. Using separately prepared FAP-expressing HEK293 cells, they performed in vitro binding assays between the CAR expressed in T cells and FAP, confirming high cytolytic activity.
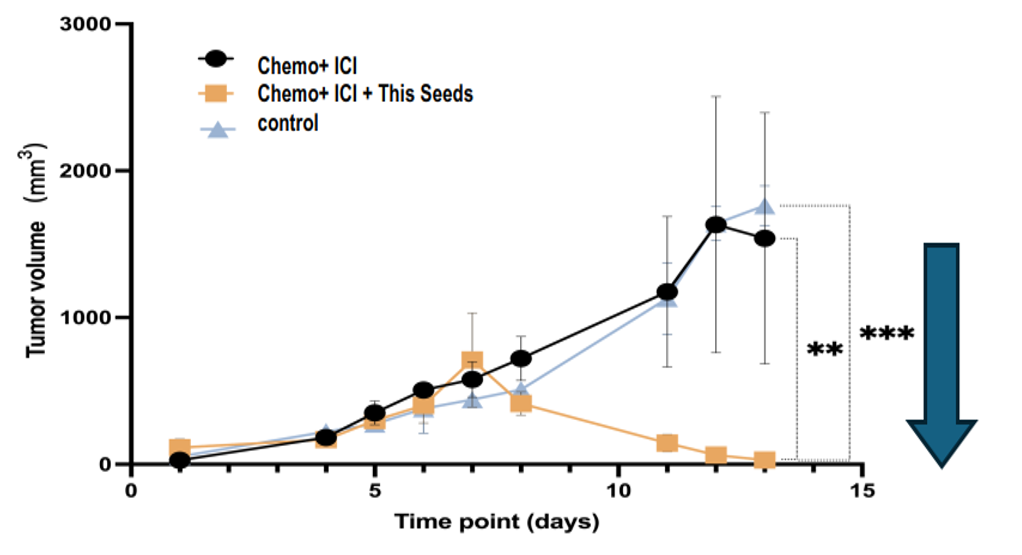
- They prepared FAP-CAR mRNA encapsulated in LNPs and administered it to CDX mice and patient-derived tumor xenograft (PDX) mice, along with various anticancer drugs and lymphocytes. The results confirmed that tumor volume growth was suppressed compared to control experiments in CDX mice harboring breast cancer-derived tumor cell lines (4T1 and E0771) and colon cancer-derived tumor cell lines (MC38), as well as in human colon cancer PDX mice. Particularly in the mouse colorectal cancer (MC38) transplant model, tumor volume was significantly reduced in each of the following test cases. In test case (b), an effect equivalent to near disappearance was confirmed (see below graph data).
(a) LNP (FAP-CAR mRNA) + 5-FU + PD-1 mAb
(b) LNP (FAP-CAR mRNA) + 5-FU + PD-1 mAb + CTLA4 mAb
(c) LNP (FAP-CAR mRNA) + 5-FU + PD-1 mAb + CTLA4 mAb + JARID inhibitor
(d) LNP (FAP-CAR mRNA) + 5-FU + PD-1 mAb + CTLA4 mAb + Small Mw cancer drug
Partnering Model
- We are seeking pharmaceutical companies willing to collaborate on verifying the cancer treatment efficacy (preclinical to clinical trials) of your company's drug candidate molecules under development when used in combination with our patented technology, through joint research with The University of Osaka.
- We are also seeking pharmaceutical companies/biotech firms possessing tissue-specific LNPs-DDS technology, as well as pharmaceutical companies willing to collaborate on elucidating the detailed mechanism of action of this invention.
- In all cases, we invite consideration of an exclusive license/option agreement for the subject invention during the joint research period.
Principal Investigator
Hideshi Ishii, MD, PhD (Professor, The University of Osaka, Japan)
Patents and Publications
Patents:
- PCT/JP2025/012812 (published in Japanese as WO2025/206323)
Publications:
- Preparing to submit a paper.