Advantages
-Disease‑exacerbating factor identified using a model that more closely recapitulates the pathological features of human pneumonia:
This model is a mouse‑adapted SARS‑CoV‑2 infection model that reproduces an acute lung injury–like pathology predominantly affecting the lung, similar to human COVID‑19. Using this model, single‑cell analysis combined with focused investigation of fibroblast populations that specifically accumulate in the severe group led to the identification of this factor as a driver of disease exacerbation.
-Potential for drug repurposing:
The ALK/ROS1 tyrosine kinase inhibitor Lorlatinib, already approved for the treatment of certain lung cancers, could potentially be repurposed as a drug candidate for severe viral pneumonia.
Current Stage and Key Data
- Current stage: Discovery / Pre‑clinical
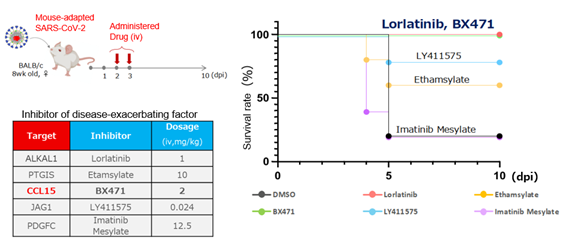
In a lethal pneumonia model induced by mouse‑adapted SARS‑CoV‑2 (rMA10), treatment with lorlatinib resulted in a statistically significant improvement in survival compared with control animals (see figure).
- Future Plan
> Plan to conduct epidemiological and translational studies to support extrapolation to humans.
> Plan of efficacy studies in additional respiratory infection models (e.g. other viruses).
> Mechanistic studies to elucidate how the identified cytokine/ligand contributes to disease exacerbation and fibroblast‑mediated lung injury.
Partnaring Model
The University of Osaka is seeking pharmaceutical companies interested in:
- Developing therapeutics based on this ALKAL1 signaling, and/or
- Engaging in collaborative research with the originating laboratory.
Direct meetings with the principal investigator can be arranged upon request. Please feel free to contact us for further information or discussion of potential collaboration.
Background and Technology
In many viral infectious diseases, fatal outcomes are thought to be driven not only by the virus itself but also by dysregulated host immune responses and the resulting irreversible tissue damage, including fibrosis. Nevertheless, the detailed mechanisms that determine progression to severe respiratory failure remain incompletely understood, and current clinical practice still relies largely on supportive and symptomatic care.
The research team established an in vivo SARS-CoV-2 infection model in wild‑type mice in which either severe or mild respiratory disease can be modulated by the viral inoculum dose, in order to elucidate the mechanisms underlying severe respiratory manifestations in COVID‑19. Single‑cell RNA‑seq was performed on lung tissues from both mild and severe groups, followed by trajectory‑based analysis to infer temporal transitions of cell fates, which led to the identification of a fibroblast subset specifically induced to differentiate in the severe group and ALKAL1 as an upstream ligand candidate.
Because fibroblast activation is increasingly recognized as a key determinant of long‑term outcomes in many severe respiratory viral infectious diseases and acute lung injury syndromes, inhibition of ALKAL1 signaling is expected to represent a promising new therapeutic strategy to prevent or attenuate progression of viral pneumonia to severe diseases.
Principal Investigator
Specially Appointed Associate Prof. Chikako ONO (Center for Infectious Disease Education and Research,The University of Osaka)
Patents
Patent application filed (unpublished).