Advantage and Core Benefit
- Different mode of action from those of existing IPF drugs, such as suppression of inflammatory cytokines and inhibition of FGFR
- Has been shown to treat not only pulmonary fibrosis but also hepatic fibrosis and is expected to expand its application to other fibroses, such as scleroderma.
Background and Technology
Idiopathic pulmonary fibrosis (IPF) is characterized by the deposition of extracellular matrix such as collagen and fibronectin by myofibroblasts differentiated from fibroblasts that have accumulated excessively. TGF-β1 is a fibrosis-promoting factor that promotes fibroblast-myofibroblast differentiation (FMD), and inhibition of FMD is one of the therapeutic targets of IPF treatment.
In the process of screening compounds with anti-FMD activity, the inventors showed that Miglustat, a glucosylceramide synthase (GCS) inhibitor clinically approved for the treatment of Niemann-Pick disease type C and Gaucher disease, inhibits TGF-β1-induced FMD by blocking Smad2/3 nuclear transfer. FMD by inhibiting Smad2/3 nuclear translocation.
In a mouse model of bleomycin (BLM)-induced lung fibrosis, treatment with Miglustat markedly improved the deterioration of respiratory function. Furthermore, the antifibrotic effects of Miglustat in the BLM-induced lung injury model were like those of pirfenidone and Nintedanib, which are clinically approved drugs for the treatment of IPF.
Data
- Knockdown of GCS in human lung fibroblast HFL1 did not suppress TGF-β1-induced FMD, suggesting that Miglustat exerts its anti-FMD effects by a mechanism independent of GCS inhibition.
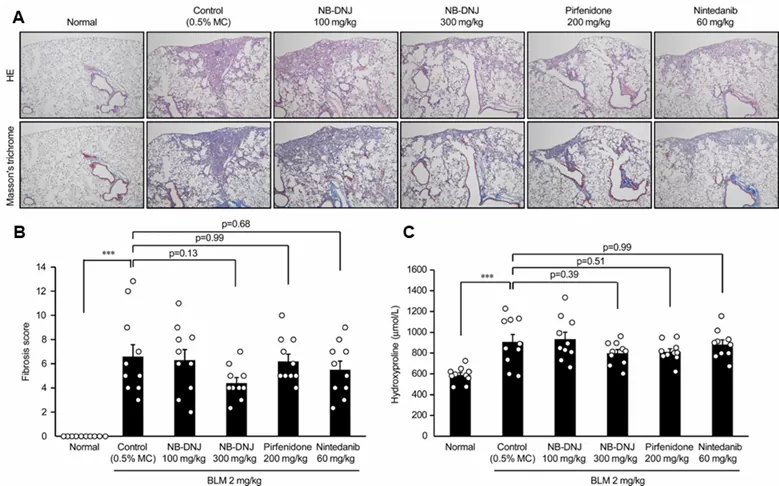
- Pathological condition of fibrosis (A), Fibrosis score (B), hydroxyproline deposition (C) after oral administration of Miglustat (NB-DNJ: 100 mg or 300 mg/kg), pirfenidone (200 mg/kg), and nintedanib (60 mg/kg) in a model of BLM-induced lung fibrosis model.
Patents and Publications
JP 7109039 (Granted), US 10,478,427 (Granted), US 10,980,78 (Granted)
https://doi.org/10.1016/j.biopha.2023.114405, https://doi.org/10.1016/j.bbrc.2022.12.025.
Principal Investigator
Dr. Hiroyuki Nakamura (Chiba University)
Expectations
Seeking companies interested in developing a drug repositioning of Miglustat for the treatment of IPF.
Project No.WL-04682