Advantages
(1) Expanded Indications: Validated efficacy not only in Hepatocellular Carcinoma (HCC) but also in Renal Cell Carcinoma (RCC), Thyroid Cancer, and Lung Cancer.
(2) Flexibility in IMPDH Inhibitors: Efficacy is not limited to Ribavirin. The mechanism has been validated with other IMPDH inhibitors, including Mycophenolic Acid (MPA) and Mizoribine, offering flexible formulation options.
(3) Applicability to Other MKIs: The sensitization effect is observed with other Multi-Kinase Inhibitors, including Sorafenib, Regorafenib, and Pazopanib, suggesting a broad platform potential.
Current Stage and Key Data
PoC Established: Proof of Concept (PoC) confirmed in both in vitro and in vivo models.
Efficacy: Combination of Lenvatinib and Ribavirin demonstrated remarkable anti-tumor effects in non-clinical trials.
Potent Synergistic Effect in vivo: In experiments using liver cancer model mice, the combination group (Lenvatinib 2 mg/kg + Ribavirin 25 mg/kg) showed significant tumor volume reduction compared to each monotherapy group.
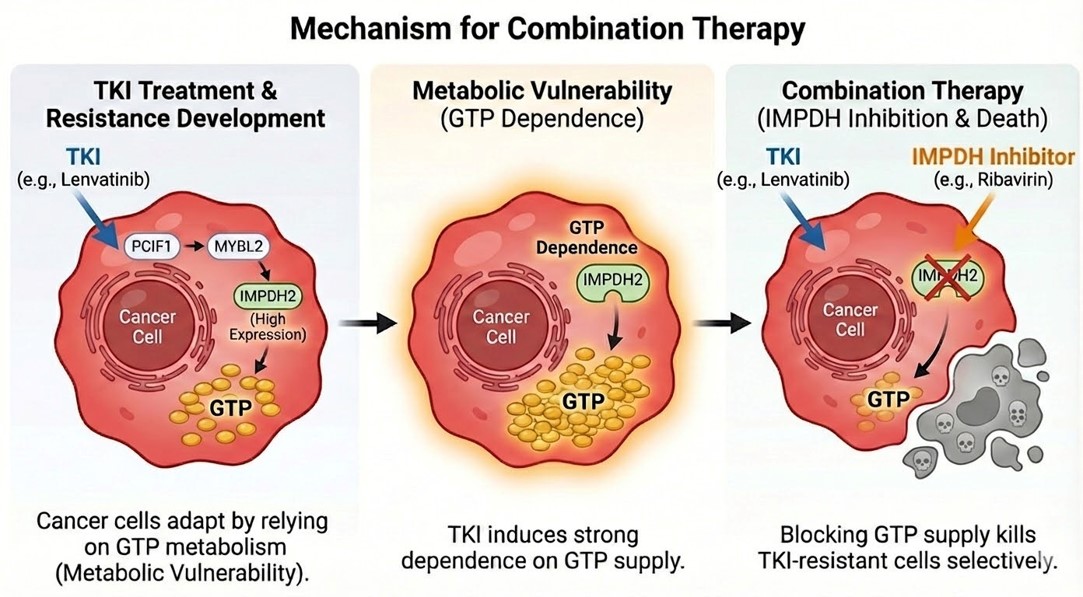
Validation of Molecular Mechanism: Confirmed that suppression of PCIF1 expression enhances Lenvatinib sensitivity. Furthermore, the fact that the effect of IMPDH2 inhibition was canceled by exogenous guanosine addition demonstrated that GTP dependency is the underlying mechanism of resistance acquisition.
Partnaring Model
(1) Target Partners Small-Molecule Oncology Drug Developers: We are seeking partnerships particularly with: Companies aiming to develop or expand indications for Multi-Kinase Inhibitors (MKIs) and Companies developing novel IMPDH Inhibitors.
(2) Joint Research & Development Evaluation & Validation of Your Compounds: Using our proprietary resistant cell lines (e.g., C3B) and evaluation systems, we can verify the anti-tumor efficacy and resistance-overcoming potential of your compounds (MKIs or IMPDH inhibitors) in combination therapy.
(3) Licensing Opportunities: We are seeking partners to enter into licensing agreements for our patent-pending technology (Combination therapy of MKI and IMPDH inhibitor) to drive development toward clinical application and commercialization.
Background and Technology
Clinical Unmet Needs: Lenvatinib is a key drug for HCC, but its response rate as a monotherapy is limited to approximately 25%. The acquisition of resistance is a major barrier to improving prognosis. Elucidating this mechanism is crucial for enhancing efficacy across various cancer types.
Proprietary Screening & Target Identification
Unique Model: Established a novel Lenvatinib-resistant HCC cell line, "C3B," derived from a NASH mouse model.
Discovery: Conducted a genome-wide CRISPR-Cas9 functional screen.
Result: Identified the mRNA methyltransferase PCIF1 and its downstream effector IMPDH2 as the key drivers of resistance among tens of thousands of genes.
Principal Investigator
Dr. Ayumu Taguchi(Nagoya City University)
Patents and Publications
Patent pending in Japan (Unpublished)