Advantages
- High Pancreatic Islet Accumulation: Lipid composition analysis confirmed approximately threefold higher pancreatic translocation compared to conventional lipid nanoparticle formulations (e.g., DSPC-LNP). Specifically, high accumulation within pancreatic islet β-cells has been confirmed.
- Potential as a Type 1 Diabetes (T1D) Treatment: Enables effective drug delivery systems (DDS) for agents that activate pancreatic islet β-cells and enhance insulin production capacity.
Background and Technology
The pancreas is located deep within the abdominal cavity and possesses a complex blood flow network, making it one of the most challenging organs for efficient drug delivery via systemic administration. Conventional lipid nanoparticle (LNP) technology, due to its inherent properties, tends to accumulate in specific organs such as the liver, making sufficient drug transport to the pancreas extremely difficult. Consequently, more effective DDS technologies are urgently needed.
To address this challenge, the researchers developed a pancreatic islet-targeting LNP technology that suppresses non-specific accumulation in organs like the liver and enables selective drug transport to pancreatic islet β-cells. By precisely controlling the particle size to approximately 150 nm, in addition to a lipid composition centered on the unsaturated fatty acid chain DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), it was confirmed that transition from the blood vessels to the pancreatic islet tissue and retention within the tissue can be maximized upon intravenous administration.
This invention enables encapsulation of diverse active ingredients within LNPs, not limited to small molecules or nucleic acids. It represents an innovative DDS platform, particularly for the fundamental treatment of type 1 diabetes, capable of transporting drugs to target pancreatic islet β-cells and ensuring reliable drug action at that location.
Furthermore, experiments have confirmed that adjusting the particle size to be even smaller, around 30 nm, enables accumulation in sites other than the islets. This makes it applicable as a DDS for exocrine system diseases such as pancreatitis and pancreatic cancer.
Key Data & Next Steps
Non-clinical proof-of-concept has been validated in mouse models. Specifically, delivery and accumulation of small molecules (e.g., nobiletin) and nucleic acid molecules (siRNA, mRNA) to pancreatic islets, along with confirmation of therapeutic efficacy, have been demonstrated.
Key Data:
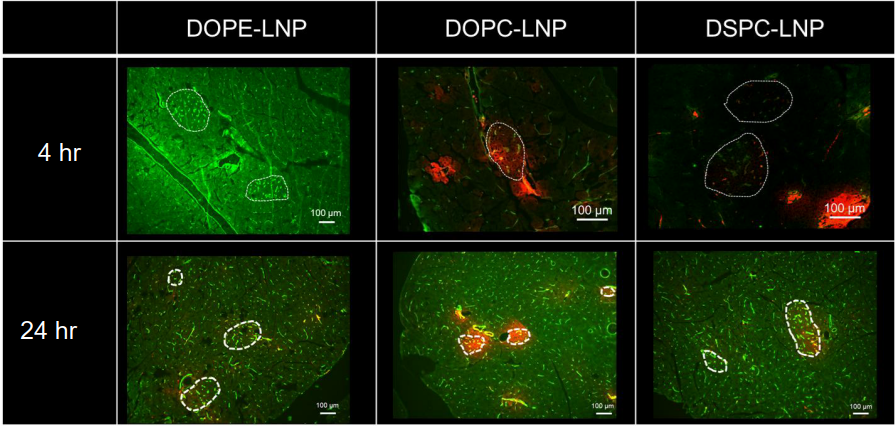
- DiD-labeled LNP was administered into the mouse tail vein. Organ distribution 24 hours post-administration was confirmed via ex vivo fluorescence imaging and pancreatic tissue section observation. Results confirmed that DOPC-LNP accumulated predominantly in the pancreas with relatively low distribution to the spleen and liver, and further demonstrated high accumulation in pancreatic islets (see figure below: dashed area indicates islets).
- When the LNP of the present invention, encapsulating nobiletin (a low-molecular-weight compound known for protecting pancreatic β-cells and promoting insulin secretion), was intravenously administered to diabetic model mice for two weeks, a significant improvement in glucose tolerance was observed at a drug dose 1/10,000 that of oral administration.
- Furthermore, experiments using LNPs containing ionizable lipids (MC3) confirmed the release of fluorescently labeled mRNA into the cytoplasm within mouse pancreatic islets, suggesting the potential for islet β-cell-specific expression induction.
Next Steps:
Researchers are planning to use LNPs encapsulating compounds known to promote pancreatic islet β-cell proliferation or mRNA therapeutics expressing proteins, and are also planning in vivo experiments (AMED-selected theme).
Expectations & Partnering Model
Tokushima University seeks collaboration with pharmaceutical companies developing treatments for human diabetes and human pancreatic diseases (pancreatitis, pancreatic cancer). Researchers also welcome joint research with companies possessing various lipid drug substances and research reagent companies. In addition to direct meetings with researchers, disclosure of non-public data is possible through signing an NDA with Tokushima University. Please feel free to contact us if you are interested.
Principal Investigator
Prof. Takanori Kanazawa, PhD
(Dept. of Clinical Pharmacology, Graduate School of Biomedicine Sciences, Tokushima University)
Patents and Publications
Patents:
- PCT/JP2024/019985 (published in Japanese as WO2024/253032)
Publications:
- Oguma T, et al., J. Control. Release (2024) 373, 917–928.
[DOI] https://doi.org/10.1016/j.jconrel.2024.07.059